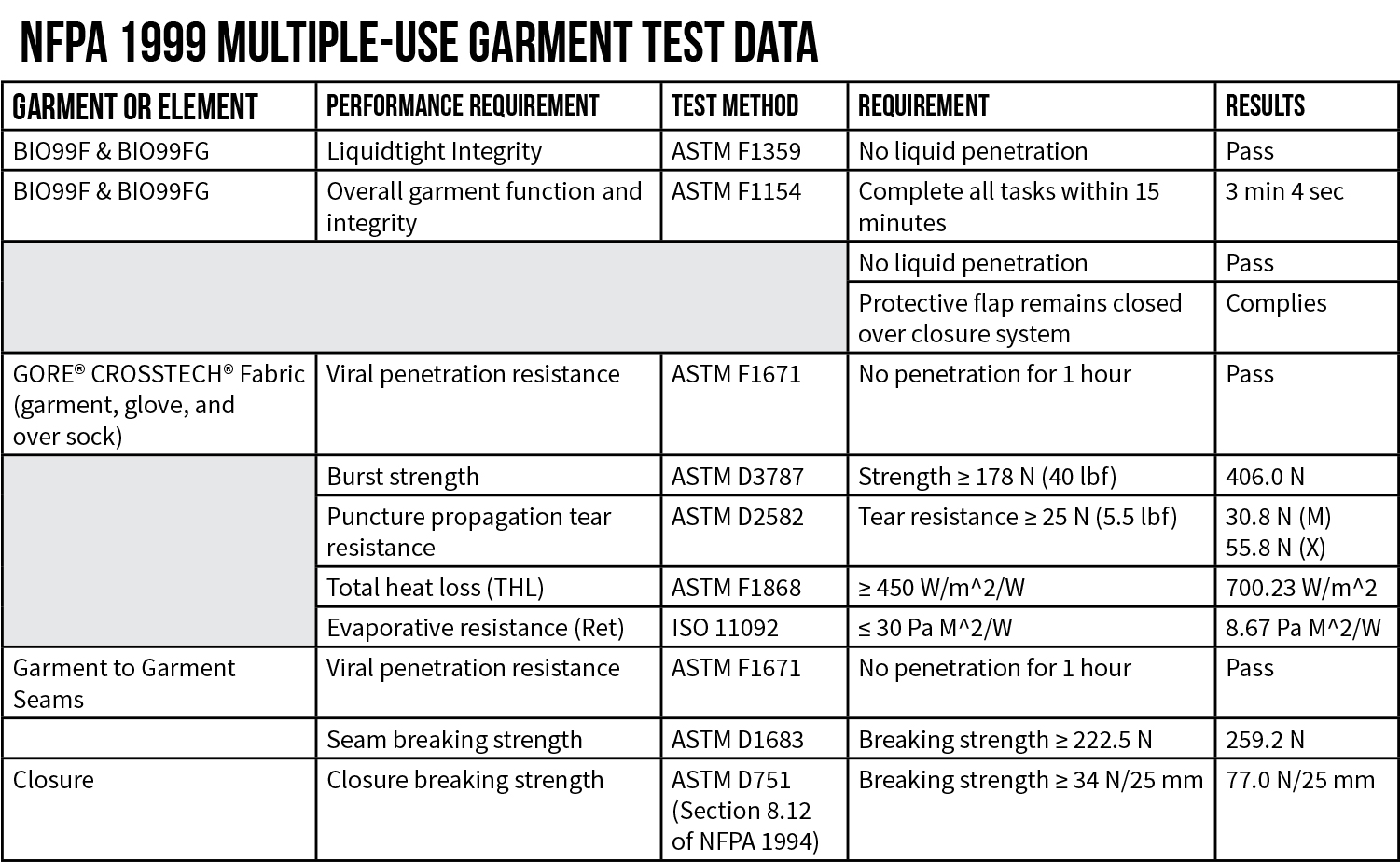
FAQ
-
Is NFPA 1999 certified PPE, such as Blauer’s BIO-99™ Coverall, appropriate for first responders and Emergency Medical personnel involved in COVID-19 response?
Yes. PPE certified to the NFPA 1999 Standard is tested against ASTM F1671 Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System. PPE compliant with ASTM F1671 and NFPA 1999 are specifically cited as meeting the InterAgency Board’s (IAB) Recommended Guidance on Protection and Decontamination for First Responders Involved in COVID-19 cases (April 10, 2020) and are also included in the International Association of Fire Chief’s (IAFC) guidance for COVID-19 PPE Decontamination.
Minimum Recommended Guidance on Protection and Decontamination for First Responders Involved in COVID-19 Cases – Detailed Reaction Guide
COVID-19 PPE Decontamination Recommendations
-
Does the Centers for Disease Control (CDC) offer guidance on the use of NFPA 1999 Coveralls like Blauer’s BIO-99 coverall by Health Care Professionals?
Yes. As part of its guidance to the Health Care Professionals (HCP) community, the Centers for Disease Control published guidance for Optimizing the Supply of Isolation Gowns for COVID-19 that includes the recommendation to consider use of NFPA 1999 certified coveralls when traditional, disposable medical PPE is in short supply. This “contingency capacity strategy” guidance cites the enhanced level of protection afforded by the 360 degree coverage a coverall offers and specifies that “In the United States, the NFPA 1999 standard specifies the minimum design, performance, testing, documentation, and certification requirements for new single-use and new multiple-use emergency medical operations protective clothing, including coveralls for HCP.
Strategies for Optimizing the Supply of Isolation Gowns
-
Does PPE certified to the NFPA 1999 standard offer a higher level of viral penetration protection than medical PPE rated to AAMI PB70 for Levels 1, 2, and 3?
Yes. PPE rated as AAMI level 1, 2 or 3 are not tested for viral penetration performance. They are only tested for various degrees of water resistance. AAMI level 3 rated garments offer “moderate water resistance” and are rated to perform better than AAMI level 1 and 2 garments, which offer “minimal to low water resistance”, respectively. PPE certified to NFPA 1999 must meet the performance requirements of ASTM F1671 for resistance to viral penetration. The ASTM F1671 test method uses a liquid test medium with a surface tension of 42 dynes/cm that is pressurized at 2 psi. AAMI level 3 garments are tested for water resistance using water rated at 72 dynes/cm and pressurized at 50 cm. Roughly speaking, the ASTM F1671 test method is performed at 3 times the pressure used for AAMI level 3 testing with liquid that has a surface tension 42% lower than the water used in AAMI level 3 testing. It is significantly more difficult to pass ASTM F1671 requirements than AAMI level 3 requirements.
-
Is Blauer’s BIO-99 coverall appropriate for use in surgical settings or for use within hospitals under typical circumstances?No. Blauer’s BIO-99 Coverall is specifically certified to the NFPA 1999 standard and is NOT an FDA-regulated medical device. It is not approved for use as PPE during surgical procedures or for use by health care professionals within hospital settings during non-pandemic scenarios when traditional, disposable medical PPE is readily available.